NOx reduction with SNCR
In order to achieve sufficiently low emissions of NOx, combustion is necessary for many fuels to take secondary purification measures. The most common method is to supply ammonia or urea (adblue) in the furnace final combustion zone, which reacts there with already formed NOx to nitrogen gas and water. In order to achieve high efficiency without the release of unreacted ammonia, it is essential to be supplied at the right temperature conditions and with an efficient mixing.
BioEld Norden AB offers complete SNCR systems. The control can be supplied as a standalone system or integrates with the boiler's existing control system. Placement of nozzles for injecting the ammonia or urea solution is selected according to the specific plant conditions.
SNCR
Under favorable conditions, ammonia (NH3) can react with nitric oxide (NO) and form nitrogen gas (N2). The technology is usually referred to as SNCR, which is the abbreviation for "Selective Non Catalytic Reduction". The desired reaction path is only possible within a narrow temperature range, which in turn is dependent on how exhausted the flue gas is. The reactions are complex and are omitted here.
Normally, it is optimal to supply ammonia to flue gases that maintain a temperature in the range 950-975oC. At too low a temperature, below about 800oC, all the ammonia supplied passes through the furnace without reacting. At an all for high temperature, above 1100oC, no reduction of nitrogen oxides (NOx) occurs, but on the contrary NOx is formed by the added ammonia. The temperature window is moved to lower temperatures the more CO contained in the flue gases. Since the temperature of the furnace varies with the load, it is necessary to be able to supply ammonia at different positions in the furnace depending on the load.
An additional requirement is that the supplied ammonia is quickly and well mixed with the flue gases. Regardless of the flue gas temperature, there is always a risk that a small part of the ammonia supplied passes through the furnace without reacting. Such ammonia is usually referred to as NH3 slip. There are usually limit values for the size of NH3 emission that is allowed. To stay below the NH3 emission limit value, the amount of ammonia added must be limited.
The process is similar if pure ammonia or urea (adblue) solution is added.
NOx formation during combustion
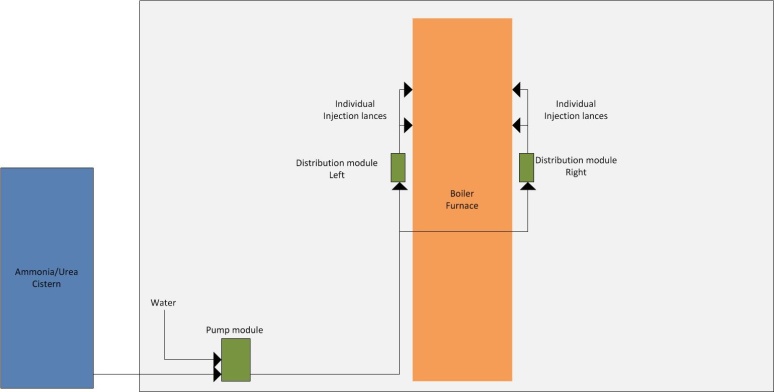
The amount of NOx formed during combustion depends on a number of different factors, including: excess air, temperature, mixing and residence time. However, when burning solid fuel, it is normally the fuel content of nitrogen that determines the plant's basic level of NOx most dominantly. The figure below shows experiential values of how the emissions of NOx are typically affected by the nitrogen content of the fuel.
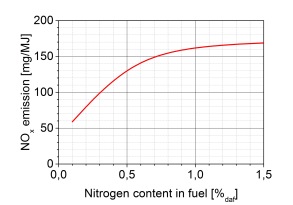
BioEld SNCR system
Ammonia solution (25%) is stored in a double-coated tank in stainless steel which is placed outside the boiler house. Alternatively, urea solution (adblue) can be used which can be stored in simpler fiberglass tanks indoors. On a ground floor indoor boiler house is placed a common pump module for ammonia / urea and diluted water (normally softened), which doses the right amount of diluted reagent to be supplied to the boiler.
The mixture is distributed to each selected SNCR lance in a distribution module located adjacent to the fireplace. When injected, the solution is atomized into small droplets using compressed air and injected at the selected level in the fireplace. Where applicable, carrier air is used to further improve mixing.
The amount of ammonia / urea added is controlled against a basic curve as a function of load. With NH3-slip regulator or NOx regulator connected, the amount is allowed to be adjusted within a given frame. The amount of diluted water is regulated so that the flow through each individual SNCR lance is always optimal.
Normally, about 50-60% reduction of NOx is obtained, but up to 80% is achieved